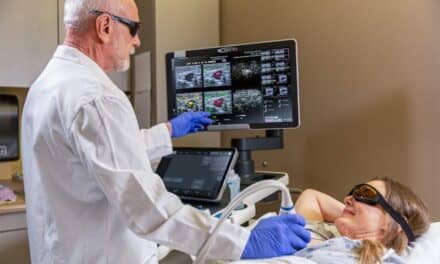
EDAP TMS has announced that its Focal One HIFU device received approval from Health Canada in January 2015. This approval authorizes the company to market the Focal One device for the treatment of prostate cancer throughout Canada.
“In the 18 months since we received EU marketing approval, the enthusiastic response from the European urology community has validated our belief in the potential of focal therapy for prostate cancer and, more specifically, our Focal One system,” said Marc Oczachowski, chief executive officer at EDAP TMS. “In a relatively short time, the technology has been adopted by some of Europe’s leading prostate cancer hospitals. We look forward to a similar reception as we establish our North American presence via the Canadian market.”
According to EDAP TMS, the focal therapy system uses accurate and MR-fused imaging to provide a noninvasive approach, along with precise and efficient therapeutic HIFU energy and end-of-treatment validation imaging with contrast-enhanced ultrasound. The EDAP TMS website characterizes the Focal One as suitable for “robot-assisted prostate tumorectomy.”
For more information about the Focal One device, visit the EDAP TMS website.