With pump quality and reliability growing ever better, pump management efficiency is the top concern for biomeds.
Like most biomeds, Mary Coker, CCE, CBET, is entirely aware of the lengthy list of potential problems she could one day see with her infusion pumps. And like most biomeds, she’s not particularly worried. “I’ve been here for two years,” says Coker, director of biomedical services at Providence Hospitals in Columbia, SC, “and to my knowledge, not once have any of our PMs detected any pump failure or calibration error.”
No, Coker says, in her day-to-day work with infusion pumps she’s primarily concerned about infection control and ensuring her techs take appropriate precautions during decontamination procedures. And then, each year, when the calendar calls for preventive maintenance, there’s that pesky issue of time—for in her experience, there never seems to be enough of it. “Just finding the pumps in the first place, considering how many are out there, is challenging, and then you have to get them all done in a single month.” The Providence Hospitals system includes two separate facilities and somewhere around 1,000 infusion pumps, Coker says, and she has four people on her team. They get the job done, but not without considerable effort. “They absolutely dread it.”
A Common Lament
As it turns out, Coker’s department is not alone. “There are a lot of hospitals out there that have 800 to a thousand pumps or more,” says Tim Ritter, CBET, CCE, senior project engineer with the Health Devices Group of ECRI Institute. “And it’s not like those pumps just stay in one place. They move around, between departments, between facilities.” Meanwhile, even when a pump is located, it’s not always the case that HTM can just take it away. “If it’s on a patient, it’s not an easy thing to switch it out,” notes Ritter.
Different facilities deal with these issue in different ways, of course. At WellSpan Health, where David Soffer, CBET, is a medical equipment I.S. specialist in the biomedical engineering department, radiofrequency identification (RFID) is used to track infusion pumps and other medical devices wherever they go. Until recently, Soffer says, he and his team relied solely on this tracking system if they needed to find a missing pump. Typically it worked, but sometimes an RFID badge would malfunction, or its battery would die, and they’d be left scrambling. (Another potential tracking issue: Many facilities that use asset trackers, Mary Coker notes, “only consider what they need to do to track their devices in the hospital itself.” She’s seen infusion pumps become temporarily lost when “people get sloppy” during patient transport and fail to change out equipment. “Unless you wire every exit door, and then have someone who’s paying attention and will actually go running” after that pump when the alarm goes off on its way out the ER, “they just walk.”)
Going forward, Soffer says, nonfunctional badges—and potential egress scenarios like the one described by Coker—should not be a problem at his facility. Following a series of recalls and safety alerts affecting their current fleet of pumps, WellSpan has decided to switch vendors. Their new pumps, slated for deployment this March, are wireless; and while they, too, will use RFID badges, that wireless capability will also allow them to be tracked through the vendor’s server. “It’s pretty slick,” Soffer says. “The pump feeds data back to the server,” which in turn produces usage reports that his team and others can use in pump management. “It might tell us how many pumps we have, how many are in use at any given time, or when and where a pump was last used,” he says. Then “administration can look at that data to see whether the number of devices we have is adequate,” or if—during the entire month of October, for example—“we only used 70 percent of our pumps, which means 30 percent were just sitting around.” Information like that is of value to any facility making purchasing decisions, but it’s also a boon to overworked biomeds. “Everyone’s always saying they can never find equipment, or there’s never enough around—well, this makes things a lot easier.”
On the flip side, Soffer predicts, because the new pumps (from CareFusion) are built on a modular platform, with a central “Point of Care Unit,” or PCU, controlling individual channels, the amount of time that is allotted for PM may feel less adequate. “We’re going from the standard single- or dual-channel pump to the situation where now each pump might have one, two, three, or four channels, and you have to hook up a laptop to that PCU to do each channel.” In the past, Soffer says, “you could set four pumps in a row and run four drips, do four occlusion-pressure tests, check your various settings, and be on your way.” Now “that’s a little more difficult because [manufacturers] want you to connect the pump to a laptop and use their software” to interface with that PCU. It’s a step-by-step process dictated by the manufacturer, leaving minimal room for the experienced biomed to speed things up through informed decision-making.
Indeed, says Shirin Khanna, senior marketing manager at Fluke Biomedical, once they’ve managed to wrangle their pumps to the shop, “most technicians will line them up and then, one pump at a time,” inspect and test according to the manufacturer’s recommendations. And those pumps that come with PM software, she notes, are not necessarily easier to service. “A lot of people are under the impression that your computer basically connects to the pump and does everything, and that’s not true. What the software does is provide a checklist of items you have to go through: Are the wires OK? Are there cracks in the pump?” And then there are the flow and occlusion readings, which can be found either manually—using a burette, stopwatch, and pressure gauge, for example—or automatically, with an electronic pump analyzer. “You’re still collecting information yourself,” notes Khanna. “The software won’t do it for you.”
A Call For Change
Which leads to the obvious question: Since, as Coker puts it, performance monitoring (or “verification”) of infusion pumps “takes up a tremendous amount of resources,” and yet all signs are that pumps being made today are sturdier and safer than ever, what can be done to make pump management easier? “As far as I can tell,” Coker says, “almost every problem you see is due to user error.” Annual pump PMs might seem like a good idea, but beyond improved and more-consistent documentation, “I’m not sure what we’re getting in return.”
Tim Ritter of ECRI Institute feels the same way. “Pumps do have some mechanical parts, but those parts are pretty reliable.” In ECRI’s role as an independent medical-device testing organization, they’ve investigated reported issues in “lots of pumps,” he says, “and we typically find that problems are use-related, not because an alarm failed or the pump was unable to deliver accurately.” In fact, Ritter says, when pumps do fail in user-related scenarios, they tend to “fail-safe”—they stop and alarm and are sent to be checked. Most such failures are not preventable, he says. “It’s usually something random and unpredictable, not something that could have been anticipated. A pump might be inspected one week and then fail the next.”
ECRI’s position, Ritter says, is that other than changing of batteries, “pumps shouldn’t require true preventive maintenance,” or those kinds of interventions typically intended to improve reliability or prevent failure. And when pump manufacturers ask that annual inspections (“performance verification”) be conducted on their devices? “It’s only a recommendation,” he says, “and it ahd to be made before their service manual was published—that’s the way it has always been.” He and others at ECRI Institute have been asking vendors to update their language to support inspection guided by manufacturer recommendations and the hospital’s maintenance experience, Ritter says. “A lot of facilities see that recommendation and read it as a requirement. They don’t want to be considered negligent”—by their own internal safety committee, or by the Centers for Medicare & Medicaid Services (CMS)—“if they don’t follow” a manufacturer’s specific instructions.
Yes, Ritter admits, infusion pumps are “high-risk devices” with mechanical parts; are typically used for many years; and have been associated with a relatively high number of adverse incidents in healthcare settings. But that doesn’t mean they should eat up the resources they typically do, especially considering the time and challenges to locate them when they’re not in use on patients. “We are aware of hospitals that have extended their pump inspection interval to two years, and in some cases, stopped scheduling inspection,” he says. HTM departments adept at capturing device data are in the best position to make such a judgment, Ritter notes. “If your pump database can give you information about past failures and whether they were preventable or not, that’s a good place to start. When possible, it’s also desirable to know that other facilities share your experience with the same model.”
As for finding pumps if they’re due for inspection, Ritter says, “most hospitals run their pumps through a central location for cleaning and redistribution. Enlist the support of the cleaning staff—just ask them, ‘Hey, if you see anything with a sticker on it that’s getting close to a year, send it over to us’” for inspection. Similarly, any pump pushed to HTM and affixed with a “broken/fix me” sign (and nothing else to indicate what’s wrong) would require a full inspection. “So think about it,” Ritter says. “Over the course of a year, you’ll probably see a significant number of pumps like this, maybe even 10 to 20 percent” of your facility’s total. “It’s the same as if you were sampling.” His recommendation: “Review your decision on inspection intervals annually, and if the problems were user detectable and not preventable, you have a strong basis for extending your interval.”
Other Considerations
Regardless of when it happens, note electronic-analyzer makers like Fluke Biomedical, pump testing must take place eventually. And when it does, they argue, their devices save time while reducing the risk of erroneous measurements. Take, for example, a facility with pumps from a variety of manufacturers. An automated analyzer, says Jack Barrett, national business development manager with Rigel Medical, provides a simple means to test all of that equipment (and up to four pumps at once) no matter the specifications for any one pump. Such devices also streamline record-keeping, he notes, so that all tests and results can be easily viewed electronically at any time. “If you ever undergo an audit and they ask you what you did, it’s all traceable and all right there.”
Beyond questions of efficiency and traceability, as wireless infusion pumps gain acceptance, and pump integration becomes more common, the main concern for HTM involves cybersecurity, notes Erin Sparnon, an engineering manager at ECRI Institute and a colleague of Ritter’s. Her suggestion: Start the security-planning process before you upgrade your pumps. “If you’re looking at an infusion-pump purchase, you first need to know what your hospital’s wireless-security-protocol requirements are,” based on the security policy your IT department has developed, “and then you need to make sure that your vendor can support” those requirements. Wireless pump servers, she adds, should be treated just like any other medical device that might transmit or holds protected health information, with pump security an integral part of the “preventive-maintenance” plan. “You might find that other departments within your facility need to be involved,” Sparnon says. “Know who has responsibility for each part of the security plan, whether it’s clinical engineering or IT” or another group, “and be sure all those departments are communicating with each another.”
At WellSpan Health, David Soffer says, their new wireless pumps, like all of their wireless devices, will rely on the WPA2-Enterprise security protocol, and so each will include a private account with its own username and password. When the pumps arrive later this winter, however, ensuring their security will be just one of his concerns. Consider, for instance, the logistical piece: “We’re getting a lot of product—close to 850 pumps—and it’s all going to be delivered to one location.” They’ll have a biomed staging area where Soffer and others will unpack the pumps as their new vendor “does their configurations and tests.” Then, he says, “they’ll hand it over to us, and we’ll do what we need to do as far as wireless networking and device identification goes.” It’s a “major project” spanning four separate facilities, Soffer notes, but when they have their new pumps—and get up to speed on their needs and requirements—it will all be worth it. “It’s kind of like getting a new car,” he says. “The interior might be different, and you might have to work to find the radio knob or the windshield wipers, but it’s still a car and it still drives.” It might take time, that is, “but you learn.”



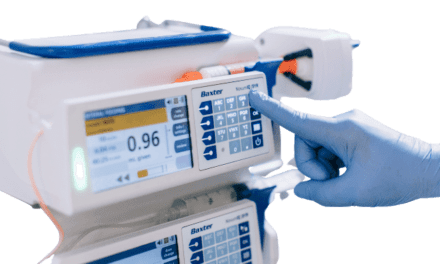
My hospital has been using the Alaris, now CareFusion, pumps for the past 10 years. Aside from tracking them down to complete the annual inspection, they are time consuming. We have an assembly line process: one tech finds and cleans, one tech will bench test the PCU (all biomed desktop PC’s have the software) one tech will test the pump module using a scale and the Fluke IDA (for pressuring testing). Finally, one tech will reconnect the pump module back to the PCU and then go back to the floors to look for more units.
It is a good process, but still takes a few months to get them all done.
Bob Tanguay/Supervisor/Elliot Hospital, Manchester, NH