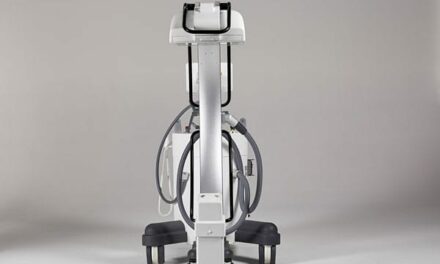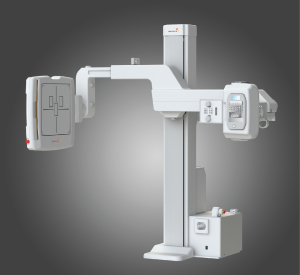
The RU-3000 has fully motorized movements for source to image receptor distance (SID), arm rotation, height, and detector angle, which can be automatically programmed to user-specific radiographic positions by either the touch screen located on the tube head, or by a handheld remote control. The system comes with a Rayence 17-by-17-inch cesium detector, which is coupled with the company’s XmaruView workstation. The RU-3000 is designed with a removable grid, patient safety anticollision sensors, and a mobile table. It is said to be well-suited for all imaging environments, especially orthopedics, imaging centers, and urgent care.
For more information about the company’s digital radiography products, visit the Rayence website.