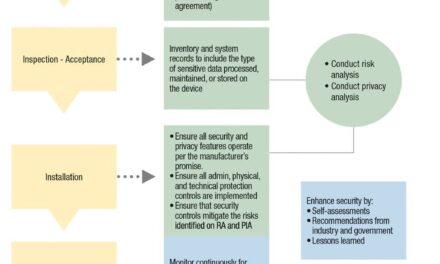
An international coalition of nonprofits, governments, and corporations involved in unique device identification (UDI) of medical devices has launched a study at Arizona’s Wickenburg Community Hospital to assess the use of scanning technology to track medical devices in the operating room.
Unique Device Identification (UDI) is a global initiative aimed at reducing medical device-related injuries and death. Each implant must be identified with a unique code that provides important information such as product, lot and serial numbers, and expiration date.
Dubbed “Blueberry Castle,” the study will assess the current manual methods of operating room implant documentation versus documenting via scanning technologies. The study will help realize international medical device data collection objectives, such as the U.S. FDA’s National Evaluation System for Health Technology and the European Union’s Medical Device Regulation programs. These initiatives are designed to use the data collected to identify opportunities to reduce healthcare costs while identifying methods to improve patient outcomes.
One of the key technologies being tested was the sterile field-scanning hardware and software, TRACTUS by Matrix IT Medical Tracking Systems Inc. Wickenburg Community Hospital is the first medical facility in the world to use TRACTUS for the documentation medical implants, instruments, and supplies used during surgery.
“Sterile field scanning will allow us to document medical implants with improved accuracy,” says Richard Wedig, chief officer of surgical services for Wickenburg Community Hospital. “The accurate data will lead to improved patient care and safety. In addition, the data collected will allow us to analyze implant performance globally which will lead to better implant/patient outcomes.”
Given the clinical and financial importance of UDI, a coalition was formed to observe and report on the results of using GS1 standards-based barcode scanning to help ensure regulatory compliance and increase patient safety, says Alan Gormley, head of industry engagement and solutions for GS1 Ireland. “The coalition includes some of the world’s top UDI stakeholders, such as GS1 Ireland, American Hospital Association’s AHRMM, Association of Perioperative Registered Nurses, U.S. and European government agencies, and others who have demonstrated a commitment to ensure the success of a global UDI rollout,” he notes.


What are the scenarios by which patient safety is increased?
Are the implants the hospital receives encoded with a serial number or batch number?
one of the really important things to use barcode scanning for in the OR is: Caregiver Scans a QR code in the room, then scans a CE# on a device, this then triggers a macro that opens a note in the chart that device has been flagged for Biomed because of a service request. This then opens a dialog box the caregiver can use to add information. A click button “sends” the request to Biomed and opens a work order in the CMMS. What once used to be a phone call or a page becomes a closed loop communication system between caregivers, biomeds, QA etc… it is 2018, what are we waiting for ???