Over the decades, improvements in occupational health and safety have come on in leaps and bounds as the level of injury, or even death, caused by hazards in our community or workplace have fallen in most developed countries to record low levels. However, better safety has come at a price. For instance, fallout from previous incidents and an increasing move toward a litigation culture has added to both the personal and financial costs associated with progress, while safety and well-being are often taken for granted.

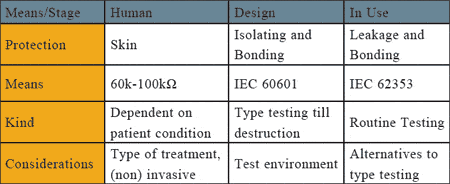
Figure 1. The different means of protection against electrical shock and how the effectiveness is tested.
On the health care front, things have also progressed, with medical procedures more successful, effective, safer, and predictable than ever before. In the future, matters will continue to improve as we look forward to even better and more efficient health care systems.
Telemedicine and home treatment are becoming increasingly common in today’s health care landscape as we see medical procedures simplified, reducing recovery times and ultimately benefiting the patient and society in return. And so it goes for medical devices—to make medical diagnoses and treatment more effective, manufacturers are continually developing more sophisticated and sensitive medical devices.
While successful treatment is the ultimate outcome, occupational health and safety must never be compromised, and so, to ensure patient and operator safety, medical device manufacturers have to comply with stringent design requirements for their medical equipment prior to introducing their products to market. Such requirements are detailed in an internationally accepted design standard called the IEC 60601 Part 1: General requirements for basic safety and essential performance.
While a medical device is deemed safe prior to its release in the marketplace, we must understand that this is no guarantee of safety during its product life cycle. Different organizations—from the manufacturer, to the operator (owner) and user—have a responsibility to maintain the equipment’s state of “fit and safe for purpose.” In order to achieve this state of fit and safe for purpose, medical device manufacturers must specify and consider factors such as:
- Intent for use (application and technical ability/limitations);
- Safe use instructions based on usability and type of users;
- (Safety) warning signs taking into account the above;
- Preventive product maintenance instructions;
- Repair maintenance instructions; and
- Instructions for disposal (subject to local codes and laws).
In Europe, the Medical Device Directive covers most of the above with the exception of testing requirements and preventive and repair maintenance requirements.
Five Years of IEC 62353
IEC 62353, its full title being IEC 62353 Medical electrical equipment—Recurrent test and test after repair of medical electrical equipment, defines the test requirements to ensure the in-service electrical safety of electromedical equipment and systems.
In meeting this requirement, the IEC 62353 incorporates tests beyond those of type testing. Specifically, it seeks to provide a uniform and unambiguous means of assessing the safety of medical devices, while maintaining the relation to IEC 60601-1 and minimizing the risks to the person conducting the assessment.
Since its release in May 2007, IEC 62353 has been widely published in most IEC member states. In Germany and Austria, the IEC 62353 has replaced the previous editions of VDE/ODE 751-1. Further afield, the Ministry of Health in Malaysia has adopted IEC 62353 (MS 62353) in its Medical Device Act as the minimum test requirement for medical electronic devices.
Even in the UK—one of only two member states that raised concerns about the concept of IEC 62353—the standard has gained popularity. In recent polls conducted at seminars, 86% of UK industry professionals were aware of IEC 62353, while 23% had already implemented it as their method of electrical safety testing. Eighty-three percent of the remaining professionals confirmed they were considering implementing IEC 62353 because of the clear benefits.
So where do we stand 5 years after initial publication? To understand this, and the benefits provided by IEC 62353, we must first look at what was happening in the medical devices industry before publication.
Notably, there was no internationally accepted standard for electrical safety testing during preventive maintenance, and few local standards existed to cover this subject. Manufacturers and hospitals that often had to refer back to the design standard (IEC 60601-1) had varying test procedures, often leading to overtesting and analyses. Furthermore, invalid testing due to different mains systems or additional grounding often occurred, and there was less time for other aspects that make up the preventive maintenance cycle.

Figure 2. To standardize the leakage measurements, a common measuring device is specified in IEC 60601 and IEC 62353. (Click image to enlarge.)
Today, leading medical device manufacturers and hospitals have incorporated the test philosophy of IEC 62353 or are in the process of doing so. Uniform test procedures and practical approaches to testing medical devices once in use have led to an unambiguous and correct approach to safety testing, giving peace of mind.
The following is a brief description of the most common tests from IEC 62353 and where their strengths have come into play.

Figure 3. Equipment leakage, direct method, class 1 equipment. L = live wire (phase); N = neutral wire (phase); PE = protective earth (ground); MP = mains parts; AP = applied parts; MD = measuring device; M = measuring device (differential method).
Ground Bond Test
A ground bond test proves the low resistance integrity between the ground pin of the mains plug and any touchable metal conductive parts on the chassis, which may become live during fault situations in Class I medical devices. Manufacturers of medical devices have welcomed the reduction in test current requirements in IEC 62353, which now specifies a minimum test current of 200mA, either AC or DC, instead of the high current 25A test in IEC 60601, which are meant to stress the protective ground path.
Unlike low test currents, high test currents do provide a practical problem during preventive maintenance, leading to potential damage to functional ground circuits and masking of poor contact resistance in older mains cabling. Protective ground problems are common, caused by either partial or complete restriction in the current path due to mechanical damage or wear and tear.
Protection Against Electrical Shock
In today’s world, it would be difficult to envisage modern life without electricity. It is taken for granted, and its benefits far outweigh the dangers. And so it must be accepted that electrical currents—referred to as functional current—are a necessary part of modern medical devices. IEC 60601-1;2006 defines leakage current as “current that is not functional,” and is an unavoidable aspect of electronic designs. However, the risk of unacceptably high leakage currents can be managed through effective levels of electrical insulation/isolation.

Figure 4. Applied part leakage, direct method, class 1 equipment. (See Figure 3 for abbreviation keys.)
Increased leakage currents can occur due to reduced insulation quality as a result of component failure (inevitable), mechanical damage leading to reduced creepage distances, evident and nonevident spillage of liquids inside the medical device, degrading of insulation materials due to aging or abrasion and environmental conditions, and unintentional misuse of the medical device.
Unintentionally high leakage currents can lead to macro shocks, current path between two different skin areas (eg, hand-to-hand or hand-to-foot), and micro shock (eg, current flow is introduced across or near the heart). A micro shock of 10?A directly across the heart can increase the risk of ventricular fibrillation to 0.2%.
Considering that electrically conductive parts can be placed in close proximity to the heart, and that electrically conductive parts from a medical device can be connected to a patient for hours, even up to several days or weeks during treatment or monitoring, the risk of micro and macro shocks cannot be understated, especially when considering that the patient might be vulnerable or have impaired ability to move or communicate.
Figure 1 provides an overview of the different means of protection against electrical shock and how the effectiveness is tested.

Figure 5. Equipment leakage, differential method, class 1 equipment. (See Figure 3 for abbreviation keys.)
Human skin acts as an electrical insulator and can limit the amount of current passing through the muscle tissues and vital organs like the heart. Body impedance (hand-to-hand or hand-to-foot) is considered 1k?, but this drops significantly when the current path is reduced, such as when the current path starts and exits closer to the heart (micro shock). In such cases, body impedance can drop to 10?.
To standardize the leakage measurements, a common measuring device (body model) is specified in IEC 60601 and IEC 62353. See the graphs, figure 2 above.
Electrical Leakage
The effectiveness of electrical insulation is tested through electric leakage measurements (results in mA or ?A).
IEC 62353 defines two different kinds of leakage current tests: The equipment leakage current and the applied part leakage current.
The equipment leakage current is the total leakage deriving from the applied parts, chassis, and mains parts combined to ground. This can also be referred to as leakage on the input of the medical device, as leakage is predominately generated in the power supply.
The applied part leakage current is the total leakage deriving from the combined patient leads within an applied part to ground and any conductive or nonconductive parts on the chassis. This can also be referred to as leakage on the output of the medical device. During this test, the effectiveness of the dielectrics of the floating applied parts is determined.

Figure 6. Equipment leakage, alternative method, class 1 equipment. (See Figure 3 for abbreviation keys.)
The strength of IEC 62353 is that leakage measurements are done primarily under single fault condition, reducing the need for repeated measurements—as is done under IEC 60601. Furthermore, IEC 62353 measurements in patient environments are always ideal. Secondary ground due to functional grounding or connections in a medical system can lead to invalid readings (false pass) caused by the internal resistance of the measuring device. Leakage currents are primarily referenced to ground, as this is the most common danger to humans. When measuring on an isolated mains supply, the line isolators in hospitals can mask high leakage currents in medical devices. As such, a false pass might occur when a medical device has generated a fault. This will only become apparent when the medical device leaves the isolated mains location.
Testing Leakage
Testing leakage under these conditions is not covered by IEC 60601 but is considered in IEC 62353 by offering different methods for testing leakage: the direct leakage, differential leakage, and the alternative methods.
The direct leakage method places the measuring device directly in the leakage current path to mimic the current path through an operator or patient. This method is a direct comparison with the IEC 60601-1 tests and can measure lower leakage values typically of less than 10?A.
However, one of the main disadvantages of this method is that the 1k? resistor (body model) is connected in series with the protective ground conductor, which will form an “equal” parallel connection with the human body, thus forming a potential hazard to the operator. Another disadvantage is that secondary ground connections will produce a lower reading, thus potentially allowing faulty equipment to pass the test. The direct method does therefore require a fully isolated Device Under Test (DUT) and must be performed on a TN (terre neutral) supply and in both polarities of the incoming mains to guarantee measurements are taken at the maximum potential leakage current. The method is applicable to both equipment and applied part leakage as shown in figures 3 and 4.
Differential and Alternative Methods

Figure 7. Applied part leakage, alternative method, class 1 equipment. (See Figure 3 for abbreviation keys.)
The differential leakage method is based on inductance and the same principles and a ground fault current interrupter (GFCI), measuring the imbalance between the line conductors. The advantage is that no additional impedance is used in the test setup, allowing the possibility to measure leakage even when a secondary earth is present. A possible disadvantage is the reduced accuracy at lower leakage values in the region of 10 to 50?A. This method is applicable to equipment leakage only. See figure 5.
Similar to an insulation test done at mains frequency and potential, the benefit of the alternative leakage method over a DC insulation test is that it gives a true indication of expected capacitive leakage, and test voltages do not exceed the design limits of the electronic designs—unlike the 500VDC used in insulation testing.
Leakage measurements achieved using the alternative method are highly repeatable and provide a good indication of deterioration in the dielectrics of the medical device under test. Typically, the alternative leakage will result in double the amount of leakage when the DUT is powered on. This is due to the mains test voltage that is present on both the live and neutral parts of the circuit.
The alternative equipment leakage provides identical results to the IEC 60601 ground leakage under open neutral fault condition and is applicable to both equipment and applied part leakage. See figures 6 and 7 above.
No Compromise
When it comes to safe and successful health care, we have come a long way thanks to global standards committees and medical professionals continuing to improve the situation on safety and well-being.
IEC 62353 has, and continues to play, a seminal role in providing an easy way to assess the electrical safety of medical devices by providing a summary of leakage tests aimed at giving a quick and easy comparison with previous and or expected reference values. And thanks to IEC 62353, electrical safety testing has become affordable, manageable, accurate, and quicker, creating more time for equally important aspects of preventive maintenance. Updates to IEC 62353 are expected in the coming years, with perhaps greater emphasis on applying the standard even as part of acceptance and end-of line production testing.
There are still ways to improve our safety and well-being by collecting and analyzing data in standard formats. This is now possible with IEC 62353, providing concise information for manufacturers and operators, which can lead to improvements in product and design and provide an important reference when evaluating new medical devices.
However, there is no resting on our collective laurels. When it comes to electrical product safety, we have to remain vigilant. While incident-reporting databases such as the FDA’s MAUDE exist in most developed countries, electrical shock incidents due to product safety are not classified as a search term and are difficult to quantify.1 The NFPA has also released a paper that concludes that fear of disciplinary action might also prevent all incidents from being reported.2Amid calls to reduce the amount of electrical safety testing in the new edition of NFPA 99, the IEC 62353 should be considered as a welcome and highly relevant standard to meet the values of NFPA 99, reducing testing times while respecting occupational health and safety.
A free guidance booklet on IEC 62353 is available for download at www.rigelmedical.com/IEC62353. Those interested in this article are also invited to join a discussion forum on IEC 62353 and its practicalities on LinkedIn. A link to this forum is provided on the Web site above.
John Backes, MA, is the associate director of Rigel Medical, Peterlee, England, a manufacturer of portable biomedical test equipment. He is active as one of the UK representatives on the IEC 60601 committee (WG14), responsible for producing and maintaining international standards for the electrical safety of medical electronic devices. For more information, contact .
References
- Smith G. Not suitable for medical use—Electrical safety testing under attack. Articlesnatch.com www.articlesnatch.com/Article/Not-Suitable-For-Medical-Use—Electrical-Safety-Testing-Under-Attack/859625. Accessed April 12, 2012.
- Chernovsky M, Sipe J, Ogle RA. Evaluation of health care operating rooms as wet/dry locations. Exponent Inc, Bowie, Md. The Fire Protection Research Foundation. www.nfpa.org/assets/files/PDF/Research/RF_OR_classification.pdf. Accessed April 12, 2012.